A priest gave me my last rites before my parents gave me a name.
This early brush with death was a result of being born six-weeks premature with pneumonia. Meaning I spent my first two-weeks within a neonatal incubator to survive.
Therefore, I was pumped full of antibiotics… the most common medication prescribed for infants in a neonatal intensive care unit. [1]
Arguably, the greatest medical breakthrough of the 20th century. Antibiotics have prevented many people from dying from infections. For one, without their existence, there is a very slim chance I’d still be alive today. Yet, it is now clear that antibiotics are not great for your internal rainforest… the gut. [2]
For instance, a 2021 study shows antibiotics create “collateral damage” by wiping out good bacteria in the gut. Leaving you open to gastrointestinal disorders and recurring infections. [3]
Likewise, a 2022 research paper highlights how a single-course of antibiotics can damage the gut of an infant. Diminishing their gut bacteria. This reduces competition and leaves more room for fungi to multiply in your gut. Throwing the gut out of its healthy balance and creating a state of, “dysbiosis.” [4]
In theory, this could allow nasty bacteria to become a dominating species. One such example could be “segmented filamentous bacteria.” This would not be good news as higher levels of this bacteria leads to changes in the lymphoid tissue. Resulting in antibodies that create a wayward immune system that damages your organs… the hallmark of autoimmune diseases like systemic lupus. [5]
No wonder I had scarlet fever three times as a child. Still together, these studies uncover a connection between gut health and lupus.
In this article we will cover why gut health is crucial in you taking control of your health. Highlighting three ways the gut bacteria differ in those with lupus in comparison to other people.
Read on to learn more.
What is Gut Flora?
First things first, what does “gut flora” mean?
The term “gut flora” refers to bacteria that live within your digestive tract. These living microorganisms help you break down the food that eat. As well as providing you with protection against harmful pathogens and toxins.
In short, they are what forms part of a healthy digestive system. Besides, providing support towards maintaining immunity levels.
Why Gut Bacteria Is Important?
The Ancient Greek physician, Hippocrates, apparently claimed that “all disease begins in the gut.”
More than 2000 years later and the role your gut health plays in causing disease is still not understood. But recent technological advances in “omics” is helping us build a better knowledge on this subject.
For example, until recently it was said that you had 10-times more bacteria than human cells in your body. Yet, science now suggests the true ratio is a tad over one microbe to every human cell. [6]
Today, you play host to over 1000 strains of bacteria. [7] But microbes and animals have co-evolved over the past 500 million years or so to become friends-with-benefits. For instance, your intestine provides nutrients to the resident bacteria you play host too. In return, the microbiome in your gut: [8][9]
- Strengthen and shape your gut
- Aid in the digestion of food and absorption of nutrients
- Produce vitamins, such as biotin (vitamin B7), folate (vitamin B9), vitamin B12, and vitamin K
- Harvest energy
- Regulate immune system function
- Hinder the colonisation of “nasty” microorganisms
For example, we are unable to digest the fibre we eat. This does not mean that eating fibre is pointless. In fact, your gut bacteria ferment this fibre to produce “short chain fatty acids.” These by-products then go on to influence the development and function of the immune and nervous systems. Having the potential to both soothe and stimulant your immune response. [10]
The belief is that your design and makeup of your gut bacteria is unique to you; Similar to fingerprints, no two people will have the exact same. [11] Even if you are a twin. [12]
What’s more, how unique your gut is may dictate how healthy you are. A 2021 research shows that older adults with a more unique gut were healthier and lived longer. Such people were able to walk faster and had better mobility. Whereas those with less unique gut societies tended to be on more medications and were twice as likely to die. [13]
This indicates that each person has their own ‘healthy gut balance’ to maintain. It is when this homeostasis is, for whatever reason, disrupted, your risk of diseases rises. [14]
– Learn more about the benefits of eating more fibre –
What is Gut dysbiosis?
Dysbiosis is a broad term for an imbalance in a microbial community.
This could be due to the gain or loss of your gut community members. Or, changes in the balance through the loss of diversity, reduction in “good” bacteria, or a gain of “bad” species. [15]
These shifts can be the result of such things like: [16]
Diet
What you eat has a big impact on the microbial composition in your gut. This in turn affects a range of metabolic, hormonal, and neurological processes. [17]
For instance, your gut bacteria changes in accordance to the food you eat. As well as the substances that the other gut microbes make. These can then affect your metabolism or cause inflammation within your body. [18] This could explain why lupus patients have an abnormal metabolism. [19]
Genetic makeup
Genes influence how your immune system functions. Including how susceptible you are to disease.
Specific “genotypes” can alter both the structures and diversity of your gut community. [20] With particular “alleles” having links with certain compositions. For example, the human leukocyte antigen (HLA) predisposes people to autoimmune disorders. With shifts in the gut microbiota influence changes in HLA structure and the underlying genetics. [21]
Inflammation/ Infections (viruses, bacterial)
Infection, antibiotic-use, or dietary changes can cause inflammation in your gastrointestinal tract. Resulting in a plethora of stressors that favour the blooming of disease-promoting species. [22]
Immune deficiency
A subgroup known as “APOBEC” plays an important role in your immune system. It helps you to defend against viruses and what science calls, internal “retroelements.” [23]
“Cytidine deaminases” is an APOBEC family member. It modulates immune responses by mutating specific “nucleic acid” sequences. Mutations or a deficiency in this enzyme can lead to changes in your gut microbiota. [24]
What does the gut bacteria look like in someone with lupus?
A persistent imbalance in your gut could fuel a number of diseases, including: [25][26][27]
- Arthritis
- Autism
- Autoimmune conditions… including systemic lupus
- Cancer
- Cardiovascular diseases
- Central Nervous System disorders
- Depression
- Diabetes
- Food allergies
- Gout
- Inflammatory bowel diseases
- Irritable bowel syndrome (IBS)
- Multiple sclerosis
- Obesity
- Periodontitis
There is no current consensus in the scientific community on what defines a “healthy” gut microbiome. Still, science suggests that certain changes have ties to systemic lupus. These include:
#1. Reduced Firmicutes-to-Bacteroidetes Ratio
Researchers believe your gut microbiota has four dominant divisions:
- Firmicutes
- Bacteroidetes
- Actinobacteria
- Proteobacteria
Science suggests that Firmicutes and Bacteroidetes represent 90% of your gut make-up.
The ratio between the two may show how healthy you are and how bad your gut dysbiosis. [28]
Most gut bacteria have their benefits. Firmicutes bacteria produce by-products that help strengthen the intestinal lining. This in turn helps prevent leaky gut syndrome. So, having lower levels of Firmicutes in comparison to Bacteroidetes has consistent ties to a collection of illnesses… including lupus. [29][30][31][32]
Still, this could be for many reasons such as female subjects in studies or medication use. For example, hydroxychloroquine lowers the diversity of gut bacteria and level of firmicutes. At the same time as increasing Bacteroidetes in mice. [33]
Hence, it remains unclear on whether this ratio is a cause or consequence of systemic lupus.
#2. Diminished Gut Diversity
Lupus patients appear to have low levels of good bacteria and more harmful ones. This is according to a recent analysis of 11 studies from 9 cities around the world.
Again, medications could play a part in this finding. With both hydroxychloroquine and glucocorticoids (think prednisone) affecting both the diversity and levels of gut bacteria. [34]
One study found that people with lupus have fewer bacterial species in their gut. But more so, the worst the persons disease was, the fewer types of bacteria they had. Implying that your lupus disease activity relates to your gut levels.
What’s more, those lupus patients had five-times the amount of one strain of bacteria: Ruminococcus gnavus (RG). While this bacterium is not harmful on its own, a high concentration may increase the risk of flares.
Also, people with active lupus nephritis show evidence of a strong antibody response to RG and its molecule “lipoglycan”. This suggests that individuals with lupus nephritis – at least – may be more prone to leaky gut syndrome, allowing lipoglycan to escape the intestines. Triggering an autoantibody response linked to kidney disease. [35]
#3. Higher levels of Segmented Filamentous Bacteria
When your immune system detects a foreign substance (“antigen”) like a virus, your white blood cells produce an antibody in response. The job of which is to bind to the “antigen” to help destroy it.
The most abundant type of antibody in your body ‘should’ be one that science calls “immunoglobulin A…” or IgA for short. It serves two important roles… to protect the mucosal tissues from infection and maintain immune homeostasis with the microbiota. [36]
‘Should’ is the key word here because some people can be IgA deficient… that is, they will have low or no IgA in their system. Most of who will be ‘healthy’ and show no symptoms. But some can develop allergies or autoimmune diseases. [37] This includes systemic lupus. [38]
Research done way back in 1983 claims that IgA deficiency is about 20-times more common in patients with systemic lupus and nephritis. [39]
Even without an infection, the body produces four grams of IgA a day. That is more than all other antibody iso-types combined. Much of this IgA ends up in the intestinal lumen, where it binds to and ‘coats’ specific members of the gut microbiota. [40]
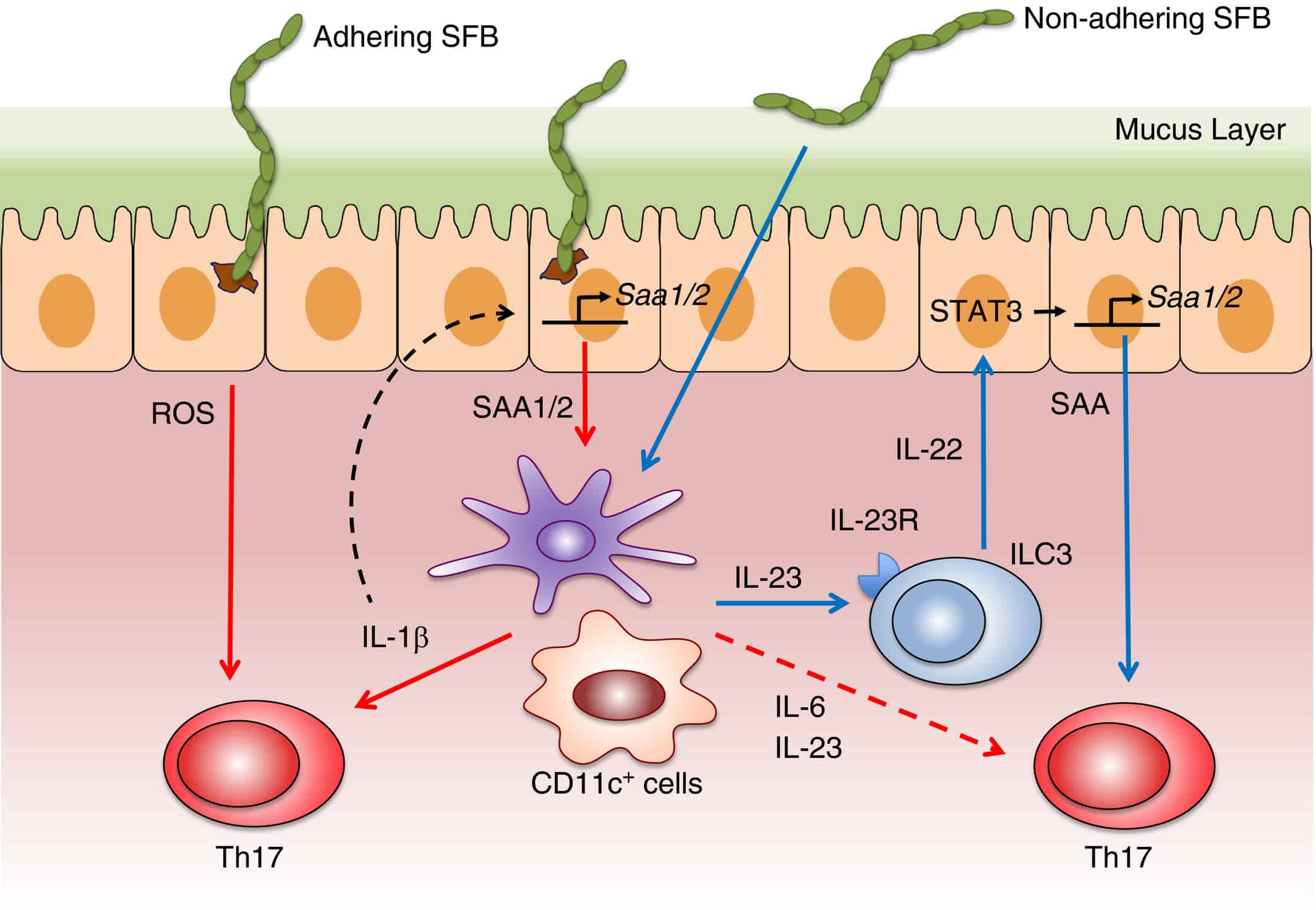
(Image cited from Flannigan & Denning, 2018)
Being IgA deficient puts you at risk of developing respiratory and/or digestive infections. What’s more, it leaves your gut open to an invasion by “segmented filamentous bacteria.” This spore-forming bacteria grow by anchoring a specialised holdfast structure to the intestinal walls. While they do not cause any direct damage to your intestines, they do have the ability activate your immune system. Stimulating the release of pro-inflammatory T cells like “Th17” and “Th1”, which can create havoc. [41]
IgA deficient mice have seen to have high levels of segmented filamentous bacteria (SFB) in their gut, leading to gut dysbiosis. [42] What’s more, exposing susceptible-mice to SFB seems to create an imbalance in their gut bacteria. While making their kidney disease and lupus nephritis worse. [43][44][45]With another research showing it can lead to antibodies that create a wayward immune system that damages your organs… the hallmark of autoimmune diseases like systemic lupus. [5]
The issue with this gut-change is that there is not much evidence out there showing that human adults have SFB in their guts. [46][47] Hence, whether SFB develop Th17 in humans is still up for debate.
Still, human studies do show that Th17 cells produce Interleukin (IL)-17, which plays a prominent role in provoking inflammation in a variety of autoimmune diseases, including lupus. [48][49] Also, taking the bacteria “Bifidobacterium adolescentis” from human gut was able to induce Th17 cells when transferred into germ-free mice. [50] Implying that even if SFB is not the main culprit, some other bacteria could be doing such a role.
— Read more: Can leaky gut trigger lupus? —
What Can You Do to Take Control of Your Health Today?
Taking charge over your wellbeing isn’t always easy. Especially if when you suffer from a condition like Lupus.
That said, there are some steps that researchers have identified that you can take to improve your gut health. These include:
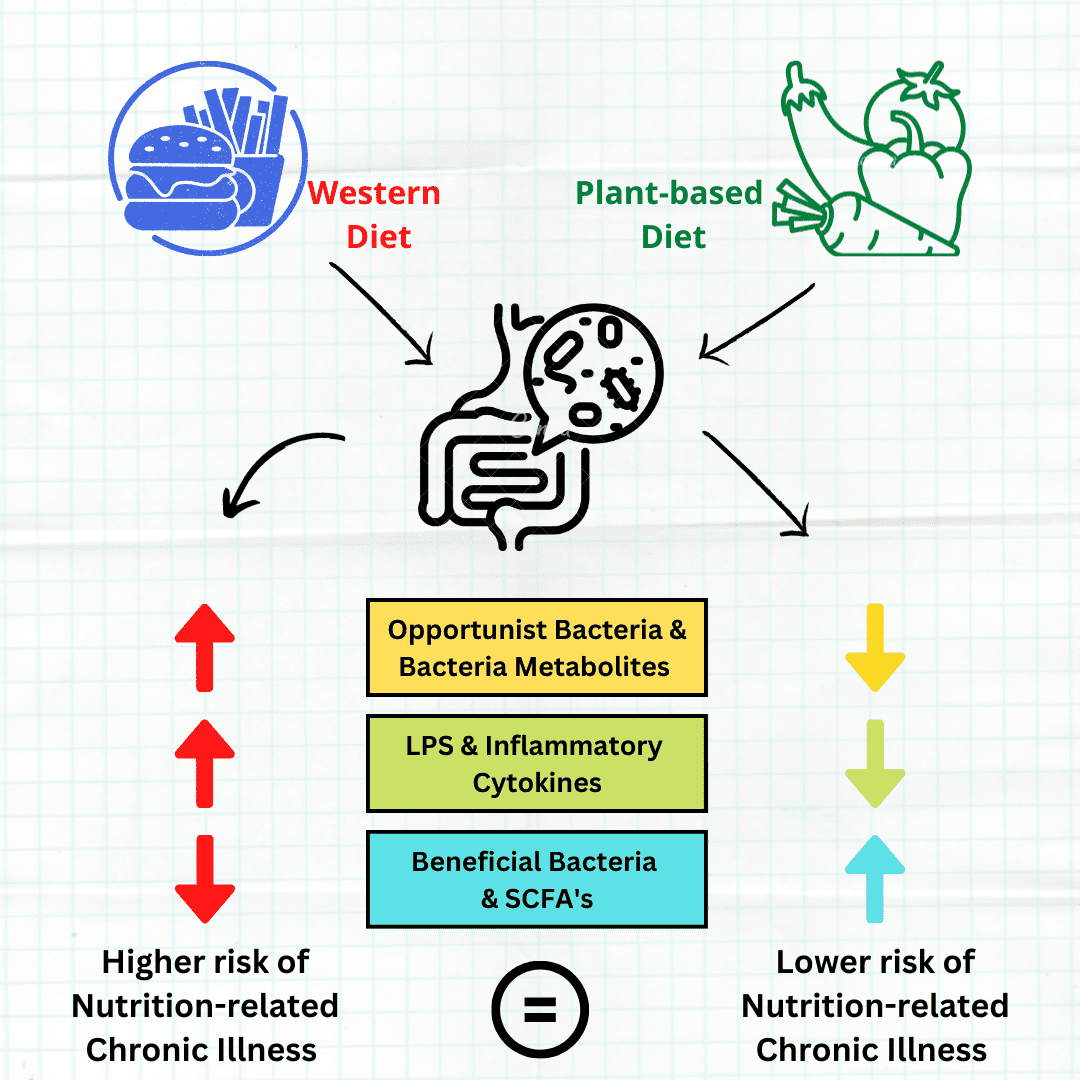
1. Change your diet
Dietary components including vitamins and fatty acids can alter your gut microbiota. [51] Hence, dietary mediated manipulation of gut microbiota is a plausible preventive task.
The typical Western diet is high on refined sugar and carbohydrates. But more so, it is low on fibre, the food of your microbiome. The best approach to restore diversity is to eat more fibre along with the bacteria that you’re missing. [52] Either through eating a diet rich in fermented foods, or probiotic supplements. [53][54]
Research shows that adopting a plant-based diet will benefit your gut microbiome. It can help to combat chronic diseases by: [55]
- Reducing inflammation
- Improving insulin sensitivity
- Promoting optimal energy balance

2. Exercise regularly
It turns out that exercise can do more than slim down your waistline and boost heart health. It might also make what’s inside your gut healthier.
Recent research published in Experimental Physiology suggests that how well your body transports oxygen to your tissues (cardiorespiratory fitness) is a far better predictor of gut diversity than either body fat percentage or general fitness activity. In the study, the researcher’s seen that those with a higher cardiorespiratory fitness had a much greater gut microbiota diversity compared to the less fit participants. [56]
Likewise, a different research paper found that those with the best cardiovascular fitness had a higher firmicutes-to-bacteroides ratio. Therefore, linking your CV fitness with your gut health. [57]
What’s more, two 2017 studies – one in mice and the other in human participants – show exercising increases the levels of short chain fatty acid producing microbes in the gut. In particular, microbes that produce butyrate… a short-chain fatty acid that promotes healthy intestinal cells, reduces inflammation and generates energy for the host. [58][59]
3. Supplement
Science suggests that you can improve your gut microbiome through consuming probiotics. [60]
Probiotics are ‘live microorganisms’ that deliver health benefits for those that take them on. Humans have been consuming these beneficial microorganisms for thousands of years. Through the likes of fermented foods.
For instance, increasing levels of “Lactobacillales” bacteria can benefit the gut. It appears to restore the mucosal barrier and reduce kidney disease. Promoting an anti-inflammatory environment – or at least it did so in lupus-prone mice. [61] Thus, long-term probiotic use may lower lupus severity by reducing inflammation and antibody production. [62]
These days, probiotics come as supplements in the form of powder or capsules. They are available, accessible and generally not expensive.
Probiotics do face the challenge of surviving the stomach environment before reaching your gut. It won’t benefit you if doesn’t reach the gut. So, to offer you the best chance of not wasting your money, remember these:
- Not all bacteria strains are as robust as one another – some will survive and some wont. Do your research before buying.
- You can buy probiotics with protective ‘enteric coating’… allowing them to survive the harsh acidic conditions your stomach provides. [63][64]
- Take your probiotic(s) with your breakfast each day, as stomach acid is naturally at its weakest in the mornings. This is then diluted even further then by the presence of food in the stomach.
- Supplement probiotics when your diet includes lots of plant-foods (vegetables, fruit, cereals etc.). These are ‘prebiotic’ foods that will feed your good bacteria and help protect them further from the harsh stomach environment.
One final note, it is not only probiotics that will alter your gut bacteria. For example, the analytic study on lupus link with the disruption of the gut microbiome found lupus patients had lower levels of “Ruminococcaceae.” [34] Research suggests that this bacterium – which is part of the Firmicutes family – will increase with inulin supplementation. [65]
Furthermore, Both N-acetylcysteine and Vitamin A treatment can influence the microbial composition and reduced the systemic autoimmunity in lupus-prone mice. [66][67]
Conclusion: Long Story Short
As my story illustrates, early antibiotic use can lead to disease due to the damage it causes to gut bacteria. This can result in a loss of keystone species, a decrease in diversity, an increase in harmful bacteria, and shifts in metabolic capacity. [68] Furthermore, certain changes have links to the onset of systemic lupus.
This situation becomes worse by the typical western diet, which fails to provide your gut bacteria with the food that it needs – ‘prebiotics’. So, one step to improving your health is to eat more fibre and more good bacteria – ‘probiotics.’ Through eating more plant/fibrous foods as well as more fermented foods or probiotic supplements. What’s more, exercising on a regular basis will help you to improve your gut health.
Understanding how much influence your gut plays in autoimmunity disorders like lupus offers hope for all patients. By making simple, yet effective lifestyle changes like eating more plant-based and fermented foods today, you can start improving your internal rainforest. Supplement this with the correct probiotics and regular exercise, you will be taking control of your health.
Pingback: How Rare is Sjogren’s Syndrome and Lupus in the UK? -
Pingback: 84 Lupus Symptoms and Signs -
Pingback: What Causes Lupus? (An up-to-date answer in 2023) -